Tungstate Radical
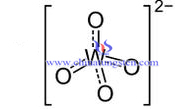
Tungstate radical is free ion in tungstic acid and tungstate solution. Tungstate radical in tungstic acid solution often appears in form of WO42- . It often exists as WO42- or (W7O246-) in tungstate solution.
In tungstate solution, the acidity gets stronger, condensation degrees of tungstate radical gets bigger. When concentrate reaches in a certain range, WO3 precipitation will be separated out from strong acidity solution, which is tungstic acid.
Organic polyamine tungstate is a new type of multi-acid compound, deca-tungstate in aqueous solution is very unstable, it will be transformed into multi-tungstate balanced mixture.
Tungstate can suppress stainless steel localized corrosion, its principle is: after WO42- moving in occlusion area will be adsorbed on the metal surface and can be fully or partially substituted adsorbed to replace chlorine ion on the surface. The corrosion products Fe2+ redox reaction of Fe3 + and WO2, tungstate and Fe2+, Fe3+ reacts hardly contain iron tungstate compound and ferrous tungstate covering the anode to form a corrosion protective film, lower occluded self caytalytic rate, thereby inhibiting further development of localized corrosion.If you have any interest in tungstic acid, please feel free to contact us by email: sales@chinatungsten.com or by telephone:+86 592 5129696.
More info>>
