Sdoium Tungstate Producing Method
Sodium tungstate producing method can be tungsten concentrate caustic leaching process, soda solution pressure digestion method.
Caustic Leaching Process
Grinding tungsten concentrate to 320mesh, add 30% caustic soda in the reactor, then reacting with calcium chloride to produce calcium tungstate, then add in hydrochloride to produce tungstic acid, sodium tungstate is produced by reacting with caustic acid then. After evaporation crystallization, centrifugal dewatering, dry then get sodium tungstate, chemical equation is:
MnWO4•FeWO4+4NaOH→2Na2WO4+Fe(OH)2•Mn(OH)2
Na2WO4+CaCl2→CaWO4+2NaCl
CaWO4+2HCl→CaCl2+H2WO4
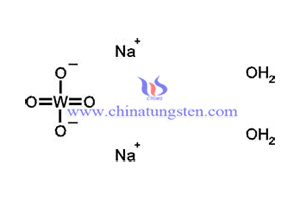
H2WO4+2NaOH→Na2WO4•2H2O

Extraction Method
Extract tungsten concentrate with sodium hydroxide, produce into coarse sodium tungstate, then react with acid and produce into tungstic acid, then by sodium hydroxide can be made into Na2WO4•2H2O.
If we need high purity sodium tungstate, we can use refining process, add sodium tungstate solution in hot high concentration hydrochloric acid, it will separate out tungstic acid precipitation. Then clean and precipitate tungstic acid for several times, dissolve it in sodium hydroxide acid, separate out and crystallize it. Under 6℃, we can get decahydrate. Over 6℃, we can get dehydrate.
Crystalline Method
1.Heat tungstic acid for 4h in 20% sodium tungstate solution, then keep it under 30~40℃ and keep mixing all the time:
H2WO4+2NaOH→Na2WO4+2H2O
2.Wait until reaction is over, filter, the filtrate is heated to concentrate and appears crystallization, cool it with ice, centrifugal dewatering, dry and get sodium tungstate reagent. Besides that, mother liquor evaporation can recycle sodium tungstate reagent with lower purity.
Soda Solution Pressure Digestion Method
Grinding tungsten scheelite and pulping, then add into the boiler with more than 1.5 times the theoretical amount in excess of soda, in complete charge was heated to 200 ~ 225 ℃, decompose for 4h. Extract was filtered, and the filtrate into the purifier after impurity removal treatment, concentration, cooling and crystallization, to obtain sodium tungstate. Chemical equation:
CaWO4+Na2CO3→Na2WO4+CaCO3
If you have any interest in sodium tungstate, please feel free to contact us by email: sales@chinatungsten.com or by telephone:+86 592 5129696.
More info>>
