Tungsten Fluoride
Tungsten fluoride is inorganic compound of fluorine and tungsten with two common forms of Tungsten (IV) Fluoride and Tungsten (VI) Fluoride, and the formulas are respectively WF4 and WF6, their basic informations are showed as bellow:
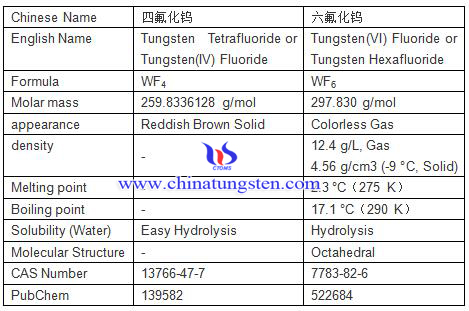
Tetrafluoride tungsten is an inorganic tungsten compound, susceptible to hydrolysis and susceptible to oxidation, and the disproportionation of it at 800℃in vacuum will generate pentafluoride tungsten and tungsten difluoride.
-fluoride-molecular-structure.jpg)
-fluoride-molecular-structure.jpg)
Tungsten(VI) fluoride, also known as tungsten hexafluoride, is the inorganic compound of tungsten and fluorine with the formula WF6. This corrosive, colorless compound is a gas under standard conditions, with a density of about 13 g/L (roughly 11 times heavier than air WF6 is one of the heaviest known gases under standard conditions. WF6 gas is most commonly used in the production of semiconductor circuits and circuit boards through the process of chemical vapor deposition – upon decomposition, molecules of WF6 leave a residue of metallic tungsten. This layer serves as low-resistive metallic "interconnect".
If you have any other question or inquiry of tungsten fluoride, please contact us through the following methods:
Email:sales@chinatungsten.com
Tel.: +86 592 5129696 / 86 592 5129595
Fax: +86 592 5129797
